Understanding Soil pH: How Phosphoric Acid Balances Nutrient Uptake
Soil pH is one of the most fundamental factors influencing plant health, yet it’s often overlooked in everyday crop and garden management. In simple terms, soil pH is a measure of how acidic or alkaline the soil is, expressed on a scale from 0 to 14. A pH of 7 is considered neutral, values below 7 indicate acidity, and values above 7 signify alkalinity. This balance plays a critical role in determining how nutrients behave in the soil and how easily plants can absorb them.
For plants to thrive, they need access to essential nutrients such as nitrogen, phosphorus, potassium, and various micronutrients. However, even if nutrients are present in the soil, an unsuitable pH can lock them away from plant roots. For example, extremely acidic soils may cause toxic levels of certain elements like aluminum, while alkaline soils can limit the availability of iron or manganese. Maintaining an optimal pH (typically between 6.0 and 7.0 for most crops) ensures that nutrients remain soluble and biologically accessible, supporting healthy growth, strong root development, and efficient fertilizer use.
One of the most effective tools for managing soil pH is the careful use of acids or bases to adjust the balance. Among these, phosphoric acid stands out in agricultural applications due to its dual role: it not only helps lower soil pH when needed but also provides a valuable source of phosphorus, a key nutrient for energy transfer and root strength. By integrating phosphoric acid into a well-designed nutrient program, growers can promote optimal nutrient uptake, enhance soil efficiency, and ultimately improve crop performance.
What Is Soil pH and Why It Matters
The pH Scale Explained
Soil pH is a scientific measure that describes how acidic or alkaline the soil environment is. It is determined using a logarithmic scale ranging from 0 to 14, where 7 represents neutrality. Values below 7 indicate increasing acidity, while values above 7 reflect rising alkalinity. Because the scale is logarithmic, each whole number change represents a tenfold shift in acidity or alkalinity, meaning that soil with a pH of 5 is ten times more acidic than soil with a pH of 6.
Although this may sound technical, the concept is straightforward: pH influences the chemical form, availability, and mobility of nutrients in the soil. Most agricultural and horticultural crops thrive in a slightly acidic to neutral range (pH 6.0–7.0), where most nutrients are readily available. When soil becomes too acidic or too alkaline, nutrient imbalances start to emerge, leading to visible deficiencies, reduced growth, and inefficient fertilizer use.
How Soil pH Affects Nutrient Availability
Soil pH directly affects the solubility of both macronutrients (such as nitrogen, phosphorus, and potassium) and micronutrients (such as iron, zinc, manganese, and copper). In acidic soils, micronutrients often become highly soluble, sometimes to the point of toxicity, while essential macronutrients may become less available. In alkaline soils, the opposite tends to occur: many micronutrients become insoluble and difficult for plant roots to absorb.
A classic example of pH-related nutrient imbalance is iron chlorosis, a condition commonly seen in high-pH or calcareous soils. In these conditions, iron becomes chemically unavailable even if it is present in sufficient quantities. Plants respond with yellowing leaves and weakened growth because iron is essential for chlorophyll production.
Another well-known issue is phosphorus lockout. In very acidic soils, phosphorus can bind tightly with iron and aluminum, while in alkaline soils it tends to form insoluble compounds with calcium. In both scenarios, plants struggle to access the phosphorus they need for root development, energy transfer, and overall vitality.
These examples highlight why managing soil pH is critical. A balanced pH ensures that nutrients stay in forms plants can absorb, ultimately improving crop health, fertilizer efficiency, and long-term soil productivity.
The Chemistry Behind Phosphoric Acid
Composition and Properties
Phosphoric acid (H₃PO₄) is a triprotic acid, meaning it contains three hydrogen ions that can dissociate in solution. This structure gives it a unique ability to react predictably within soil environments. Commonly produced from phosphate rock, phosphoric acid is widely used in agriculture as both a fertilizer component and a soil amendment. In liquid fertilizers, it serves as a concentrated source of plant-available phosphorus, while in soil conditioning products, it is valued for its controlled acidifying effect.
Unlike stronger mineral acids, phosphoric acid is considered a moderately strong acid, which makes it easier to handle and safer to apply in agricultural contexts. Its ability to release hydrogen ions in a stepwise manner (rather than all at once) gives farmers and growers more precision when adjusting soil or nutrient solution pH.
Why Phosphoric Acid Is Effective in Soil Management
The effectiveness of phosphoric acid in soil management stems from its gradual dissociation. As it enters the soil, H₃PO₄ releases its hydrogen ions in stages (H₂PO₄⁻, HPO₄²⁻, and PO₄³⁻), each contributing to a controlled reduction in pH. This stepwise dissociation prevents the sudden drops in acidity that can occur when applying stronger acids, reducing the risk of root burn or microbial disruption.
Additionally, phosphoric acid directly contributes to the soil’s nutrient profile. Its dissociation products - primarily dihydrogen phosphate and hydrogen phosphate - are immediately usable forms of phosphorus, an essential macronutrient. This dual action means growers can correct pH imbalances while simultaneously improving phosphorus availability.
Another advantage is pH stabilization. Because it reacts gradually and forms stable phosphate compounds in the soil, phosphoric acid tends to maintain a more consistent pH without overshooting into overly acidic conditions. This makes it an effective tool for long-term soil management, particularly in systems where precise nutrient uptake is critical, such as high-value crops, fertigation programs, or controlled-environment agriculture.
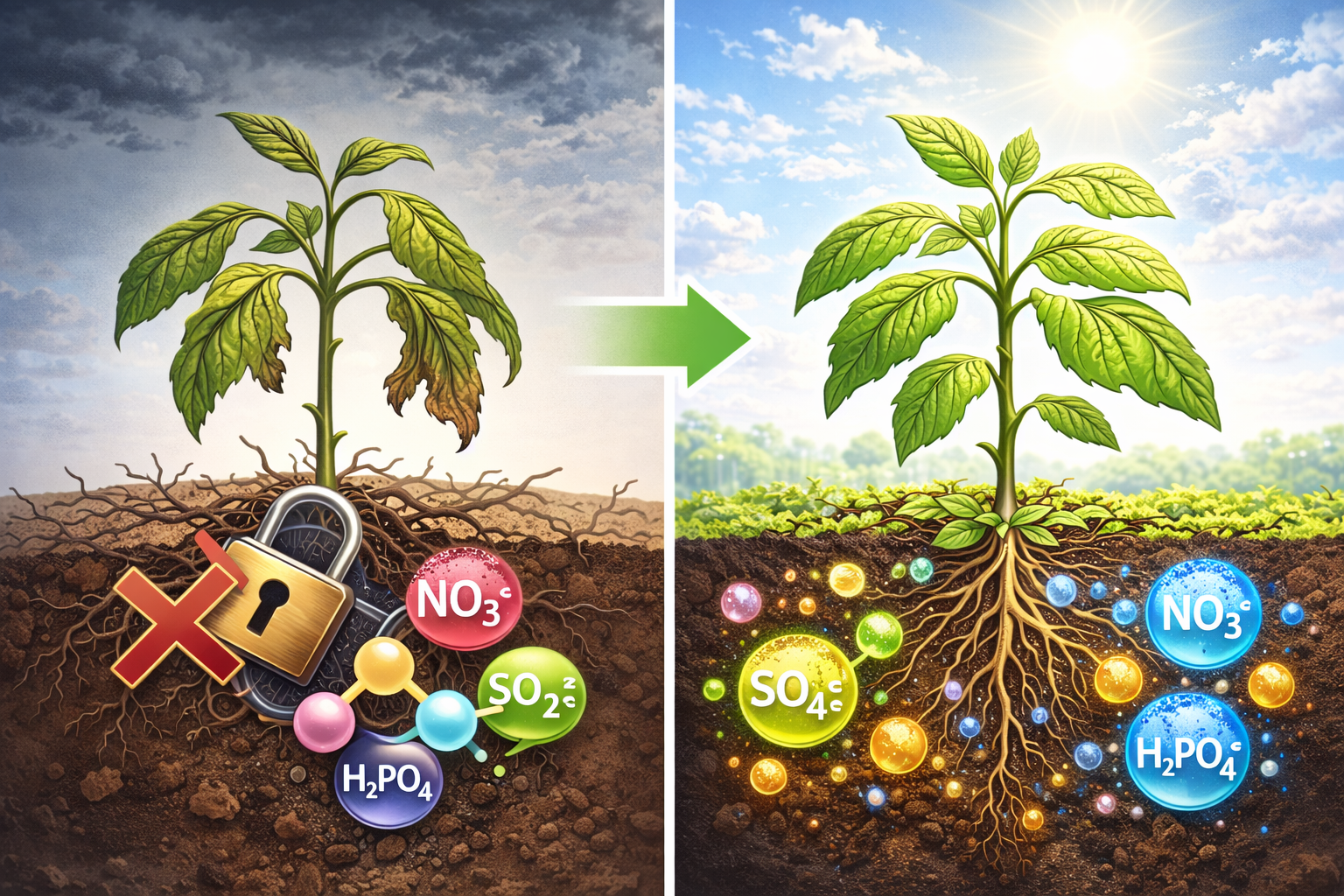
How Phosphoric Acid Balances Nutrient Uptake
Increasing Nutrient Solubility
One of the most valuable roles phosphoric acid plays in crop management is its ability to enhance nutrient solubility, especially in soils where pH is too high for optimal plant uptake. At elevated pH levels, many nutrients form insoluble compounds that roots cannot absorb. By carefully lowering the pH, phosphoric acid reactivates these nutrients and brings them back into a plant-available form.
Phosphorus availability is a key example. In alkaline soils, phosphorus tends to bind with calcium, forming stable compounds that are unusable to plants. When phosphoric acid is applied, the resulting shift toward a slightly acidic environment helps break these bonds, converting phosphorus into soluble dihydrogen phosphate (H₂PO₄⁻) and hydrogen phosphate (HPO₄²⁻) - the forms most readily taken up by plant roots. This makes fertilization far more efficient, reducing waste and improving crop response.
Micronutrients such as iron, manganese, and zinc also become more soluble when the soil is gently acidified. In high-pH soils, these elements often precipitate or become chemically locked away. By lowering the pH into the optimal range, phosphoric acid reactivates these essential micronutrients. This helps prevent common problems such as iron chlorosis, manganese deficiency, or zinc-related stunting, issues frequently seen in fruit trees, ornamentals, and row crops grown in calcareous soils.
Preventing Nutrient Lockout
Nutrient lockout occurs when the soil’s chemistry prevents plants from absorbing nutrients that are otherwise present. High-pH alkaline soils are particularly prone to this, as many nutrients become unavailable above pH 7.5–8.5. Phosphoric acid helps counteract this by adjusting the pH just enough to restore nutrient mobility without causing excessive acidity.
In contrast to strong acids, phosphoric acid’s controlled dissociation means it acidifies predictably. This allows growers to prevent overcorrection - an issue that can create its own nutrient imbalances, such as aluminum toxicity or rapid nitrogen mineralization. With phosphoric acid, pH can be managed within a stable, plant-friendly range that supports balanced nutrient uptake across all growth stages.
Case Examples
Greenhouse crops - such as tomatoes, peppers, cucumbers, and ornamentals - benefit greatly from precise pH control. Because greenhouse media often have limited buffering capacity, even slight pH shifts can influence nutrient uptake. Growers frequently use phosphoric acid in fertigation systems to maintain a stable pH in irrigation water, ensuring that micronutrients stay soluble and fertilizers perform at maximum efficiency. This results in stronger root systems, improved fruit set, and more uniform growth.
In hydroponics, pH control is even more critical. Plants rely entirely on the nutrient solution for access to minerals, and pH must be continuously maintained within the optimal range (usually 5.5–6.5). Phosphoric acid is widely used as a primary pH-down agent because it stabilizes solutions and contributes usable phosphorus, making it both corrective and nutritive.
In traditional soil systems, phosphoric acid is used more selectively, especially in alkaline or calcareous regions. Here, its role is to fine-tune pH around the root zone, improve fertilizer efficiency, and unlock micronutrients that would otherwise remain unavailable. When applied correctly, it supports long-term soil fertility and more resilient crop performance across a wide range of field conditions.
When and How to Use Phosphoric Acid in Soil
Application Methods
Phosphoric acid can be applied in several ways depending on the crop type, growing environment, and the severity of the pH issue. The most common method is through watering, where the acid is diluted in irrigation water and applied directly to the root zone. This approach provides even distribution and allows for gradual pH adjustment, making it ideal for growers who want steady, controlled improvements in nutrient availability.
Another popular technique is fertigation, where phosphoric acid is injected into drip systems or irrigation lines. This method offers high precision and is widely used in commercial agriculture and greenhouse production. Because fertigation delivers both water and nutrients simultaneously, it ensures that plants receive consistent doses of phosphorus while the soil pH is adjusted.
For certain crops, foliar sprays may be used to supply phosphorus directly to leaves, especially when root uptake is temporarily impaired due to high pH. While foliar application doesn't significantly change soil pH, it can help correct micronutrient deficiencies that are indirectly caused by pH imbalances. However, foliar sprays should complement, not replace, root-zone management.
Dosage and Safety
Proper dilution is essential when working with phosphoric acid. Concentrated acid should always be diluted before application to prevent root burn, leaf damage, or adverse soil reactions. Most agricultural uses involve adding small, measured amounts to irrigation water, often in the range of a few milliliters per liter, depending on concentration and desired pH adjustment. It is important to follow manufacturer guidelines or consult an agronomist for precise dosing.
Safety should never be overlooked. When handling phosphoric acid, growers should wear gloves, goggles, and protective clothing. Storage containers must be kept sealed, upright, and away from extreme temperatures. Always add acid to water, not the other way around, to avoid splashing or excessive heat generation during dilution.
Testing Soil pH Before Application
Before adjusting soil pH, it is crucial to test it accurately. A variety of tools are available, each with its own level of precision.
pH meters are the preferred method for growers who need consistent and accurate readings. These digital devices provide rapid measurements and are ideal for greenhouses, hydroponics, and field monitoring.
Lab tests offer the highest reliability. Soil samples can be sent to agricultural laboratories for full nutrient and pH analysis, allowing growers to make informed decisions about phosphoric acid use and overall fertilization strategy.
For quick, on-site assessments, DIY kits - often using color-changing strips or solutions - can provide a general indication of soil acidity or alkalinity. While less precise, they’re useful for routine checks or for gauging whether a more detailed test is necessary.
By combining proper testing, careful dosing, and appropriate application techniques, phosphoric acid becomes a powerful tool for maintaining optimal soil chemistry and ensuring efficient nutrient uptake.
Alternatives to Phosphoric Acid for Adjusting Soil pH
While phosphoric acid is an effective and dual-purpose tool for lowering soil pH and supplying phosphorus, growers have several other options depending on their crop needs, soil conditions, and management style. Each alternative comes with its own advantages and limitations.
Elemental sulfur is one of the most common long-term solutions for acidifying soil. When applied, soil bacteria convert sulfur into sulfuric acid, gradually lowering pH. Its main strength lies in its slow, controlled action, making it suitable for large fields or perennial crops where steady pH adjustment is preferred. However, this microbial conversion requires warm, moist conditions and can take weeks or months, so sulfur is not ideal when immediate pH correction is needed.
Citric acid, an organic acid derived from citrus fruits, offers a more natural and environmentally friendly alternative. It works quickly, making it helpful for short-term adjustments, container plants, or organic growing systems. However, its effects are temporary, as citric acid breaks down rapidly in soil. It is best used for minor corrections or as a supplemental tool rather than a primary pH management strategy.
To raise soil pH, dolomite lime (a calcium-magnesium carbonate blend) is the most widely used amendment. Dolomite not only neutralizes acidity but also adds calcium and magnesium, nutrients essential for cell structure, chlorophyll formation, and overall plant vigor. The downside is its slow reaction rate; it may take months to see the full effect, and overapplication can be difficult to reverse.
Choosing the right alternative depends on the urgency of the correction, crop type, and whether additional nutrients are desired. Each option can be effective when matched properly to the soil’s needs.
Common Mistakes and How to Avoid Them
One of the biggest risks when using phosphoric acid is over-acidifying the soil. Because acidification can happen quickly, especially in sandy or low-buffer soils, adding too much can push the pH below the optimal range. This not only reduces nutrient availability but can also harm beneficial microbes and root systems. The best prevention is to apply phosphoric acid gradually and monitor the pH over time rather than making large adjustments all at once.
Another common mistake is applying phosphoric acid without testing pH first. Soil pH varies significantly from one area to another, and guessing can lead to misapplication or unnecessary amendments. Always test the soil before adding any pH-adjusting product. Even a simple at-home test can provide enough insight to guide proper dosing and avoid costly errors.
A third issue arises from using poor-quality or contaminated phosphoric acid products. Low-grade or impure acids
may contain unwanted residues or heavy metals that can accumulate in soil or affect plant health. For agricultural use, it’s important to select products specifically formulated for fertilizer or soil management applications, preferably with clear labeling, known purity, and reliable sourcing.
By understanding these common pitfalls and taking simple precautions - testing, applying carefully, and choosing high-quality inputs - growers can safely and effectively use phosphoric acid to optimize soil conditions and support healthy, productive crops.
Frequently Asked Questions (FAQ)
How quickly does phosphoric acid change soil pH?
Phosphoric acid typically begins adjusting soil pH immediately after application, especially when applied through irrigation or fertigation. In well-aerated, low-buffer soils (such as sandy mixes or potting substrates), the change can be observed within hours. In heavier or high-buffer soils, the effect may take several days as the acid reacts with soil minerals and stabilizes. Because its action is relatively fast compared to sulfur or lime, it’s important to monitor pH frequently after application to avoid over-acidification.
Is it safe for all plants?
When used correctly and at proper dilution rates, phosphoric acid is safe for most crops, including vegetables, ornamentals, greenhouse plants, and fruiting species. However, plants adapted to alkaline soils, such as lavender, rosemary, and some succulents, may not respond well to sudden drops in pH. These species prefer stable, higher-pH conditions. Always adjust pH gradually to avoid stress, and tailor application rates to the specific crop requirements.
Can phosphoric acid harm soil microbes?
In moderate amounts, phosphoric acid does not typically harm beneficial soil microbes. In fact, correcting overly alkaline conditions it can create a more favorable environment for microbial activity. Problems arise only if the soil becomes too acidic, which can suppress microbial diversity and reduce natural nutrient cycling. This is why controlled dosing and frequent pH testing are essential - maintaining soil within the optimal range supports both plant roots and microbial communities.
How often should soil pH be tested?
For most growers, testing soil pH two to four times per year is sufficient. Intensive production systems - such as greenhouses, hydroponics, or high-value specialty crops - benefit from more frequent monitoring, sometimes every one to four weeks. Testing should always be done before applying phosphoric acid or any other pH-adjusting amendment, and follow-up tests are recommended after application to ensure the pH stabilizes within the target range. Regular testing helps prevent nutrient lockout, maintains fertilizer efficiency, and supports long-term soil health.
Conclusion
Maintaining a balanced soil pH is essential for healthy plant growth, efficient nutrient uptake, and long-term soil productivity. Even when soils contain adequate nutrients, an imbalanced pH can lock them away from plant roots, leading to deficiencies, stress, and reduced yields. That’s why understanding and managing soil pH should be a core part of every grower’s strategy.
Phosphoric acid offers a practical and effective solution for adjusting high-pH soils while simultaneously supplying a valuable source of plant-available phosphorus. Its controlled acidifying action, predictable behavior, and dual benefits make it a reliable tool for growers in both traditional and modern production systems.
To get the best results, always test soil pH before making adjustments, apply phosphoric acid gradually, and use high-quality products designed for agricultural use. With careful management, growers can maintain optimal soil chemistry and support healthier, more productive crops year-round.
















