The Hidden Ingredient Behind Clean Water: How Nitrates Are Used in Wastewater Treatment and Environmental Protection
Clean water isn’t just about getting rid of visible waste — it’s also about removing what we can't see. Beneath the surface of your tap water, a complex network of chemical and biological processes ensures that harmful compounds are neutralized or removed altogether. One of the lesser-known yet critical players in this process? Nitrates.
Often discussed in the context of pollution, nitrates (NO₃⁻) actually serve a crucial function in the controlled environment of wastewater treatment. When managed properly, they help purify water, reduce environmental harm and maintain ecological balance. As our world grapples with the twin challenges of urbanization and climate change, understanding the role of nitrates has never been more important.
What Are Nitrates and Why Do They Matter?
Nitrates are chemical compounds made up of nitrogen and oxygen (NO₃⁻). Naturally occurring in soil and water, they are essential to the Earth's nitrogen cycle — a process that helps regulate plant growth and maintain environmental balance. However, human activities have greatly increased nitrate concentrations in the environment.
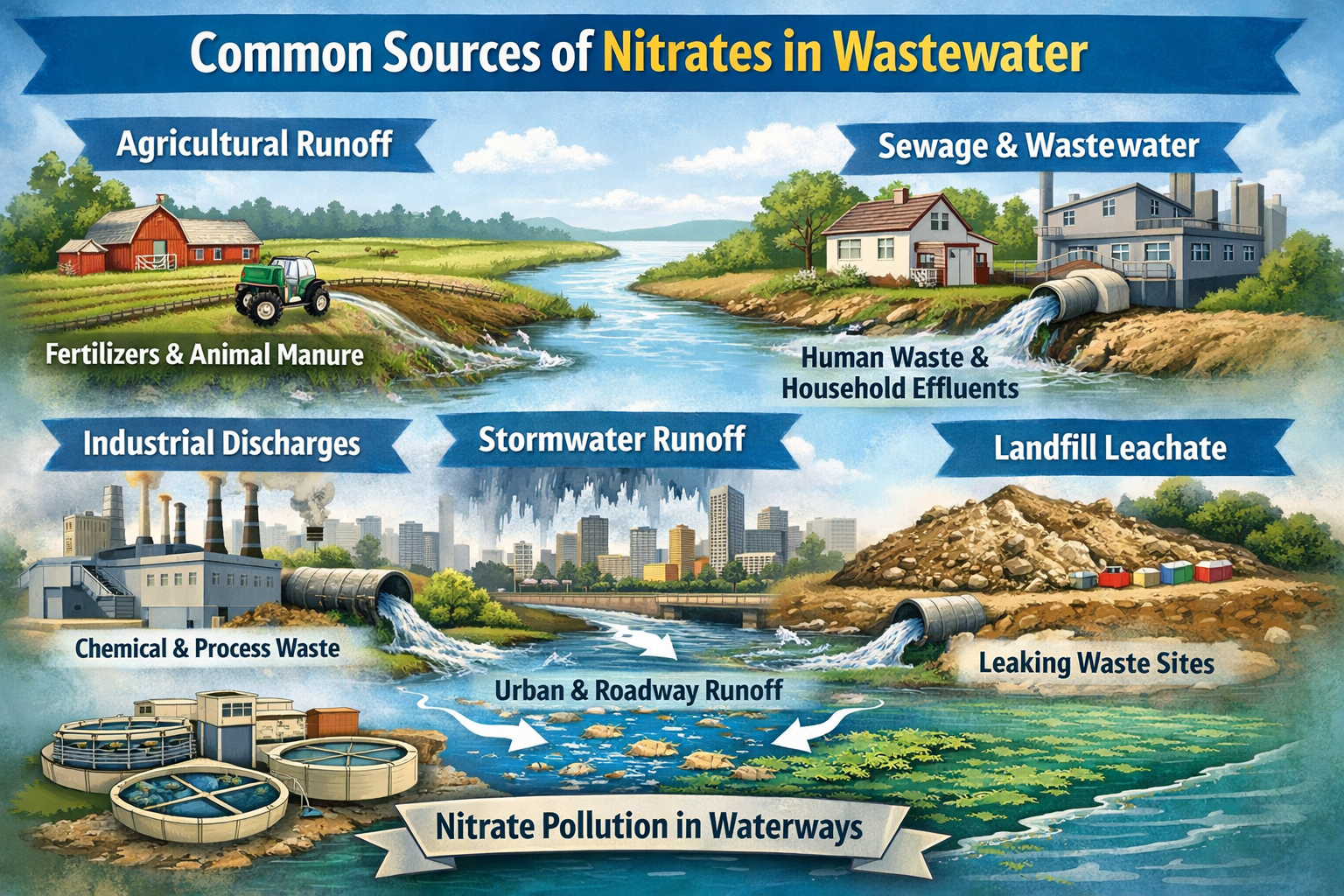
Common Sources of Nitrates in Wastewater
- Residential runoff: Lawn fertilizers and cleaning products
- Agricultural sources: Manure and synthetic fertilizers
- Industrial discharge: Certain manufacturing and chemical processes
- Septic systems: Leaky or unregulated systems can leach nitrates into groundwater
The Science Behind Nitrates in Wastewater Treatment
In modern wastewater treatment, nitrates play a key role in biological nutrient removal (BNR), particularly in managing excess nitrogen — one of the most common water pollutants.
Step-by-Step: How Denitrification Works
- Nitrification: Ammonia (NH₃) in wastewater is first oxidized into nitrites (NO₂⁻) and then into nitrates (NO₃⁻) by aerobic bacteria.
- Denitrification: In anoxic conditions (absence of oxygen), specific bacteria utilize nitrate as an alternative electron acceptor, converting it to harmless nitrogen gas (N₂), which is released into the atmosphere.
Why Too Much Nitrate Is a Problem
Excessive nitrates can lead to eutrophication — a rapid growth of algae that suffocates aquatic life. In humans, high nitrate levels in drinking water have been linked to serious health risks, including blue baby syndrome.
How Nitrates Help Clean Our Water Systems
Despite the risks of overexposure, controlled nitrate use in wastewater treatment is a powerful tool for environmental protection.
- Nitrogen pollution reduction: Proper nitrate management helps prevent the accumulation of harmful nitrogen compounds.
- Electron acceptors: In anoxic zones of treatment plants,
nitrates are intentionally added to fuel denitrification.
- Process optimization: When applied correctly, this process enhances overall treatment efficiency.
Case Study: Nitrate-Based Wastewater Treatment Systems
In several European municipalities, including regions in the Netherlands and Germany, nitrate-enhanced systems have significantly improved effluent quality. These advanced systems are often supported by specialized suppliers, such as DECACHEM, a company known for providing high-quality nitrates and other reactive chemicals that meet strict environmental and safety standards.
Environmental Benefits of Nitrate Management
When nitrates are properly regulated, they contribute to healthier ecosystems and safer communities.
- Ecological restoration: Balanced nitrate levels help restore wetlands, rivers, and lakes.
- Combatting hypoxia: In areas like the Gulf of Mexico, better nitrate control is reducing oxygen-depleted “dead zones.”
- Soil and groundwater protection: Avoiding nitrate overuse prevents long-term soil degradation and groundwater contamination.
How It Supports Environmental Regulations
Laws like the Clean Water Act in the U.S. and similar EU directives set strict nitrate discharge limits. Facilities must monitor and manage nitrate levels to comply with EPA guidelines and local environmental standards.
Challenges and Controversies Around Nitrates
Not all nitrate use is beneficial — particularly when it’s uncontrolled.
- Agricultural runoff remains a significant source of pollution.
- Excess nitrates can
accumulate in water supplies, creating health hazards.
- Debates continue around the role of industrial farming and the need for stricter regulation.
Innovations in Nitrate Use and Wastewater Treatment Technology
New technologies are making nitrate management more sustainable and efficient:
- Smart sensors and AI: Monitor nitrate levels in real-time
- Biofiltration and wetlands: Natural systems help reduce excess nutrients
- Green infrastructure: Cities are investing in nitrate-friendly stormwater solutions
What You Can Do: Supporting Clean Water Efforts
- Reduce fertilizer use: At home and in your community
- Support local policies: Back initiatives that promote better wastewater infrastructure
- Stay informed: Advocate for responsible chemical management
Frequently Asked Questions (FAQs)
Are nitrates harmful or helpful in water treatment?
Both. In controlled settings, they help remove nitrogen. In excess, they become a pollutant.
How do wastewater plants control nitrate levels?
Through processes like nitrification and denitrification, using bacteria and controlled oxygen conditions.
Can nitrate-treated water be used for agriculture?
Yes, once cleaned, treated water may be used for irrigation, especially in areas with water scarcity.
What happens if nitrate levels are too high in the environment?
It can lead to algae blooms, oxygen depletion in water bodies, and health risks in drinking water.
Is nitrate removal expensive for municipalities?
Initial costs can be high, but long-term environmental and health benefits often outweigh the investment.
Conclusion
Nitrates are more than just potential pollutants — when managed wisely, they are powerful tools in our effort to keep water clean and ecosystems healthy. Balancing their use in wastewater treatment is critical to protecting both people and the planet.
Companies like
DECACHEM play an important role in this effort, supplying high-grade nitrates and supporting industries committed to environmental responsibility. Whether you’re a policymaker, an engineer, or a concerned citizen, understanding how nitrates work is a step toward securing a cleaner, safer water future for all.