The Great Salt Confusion: What’s the Difference Between Sodium Nitrate and Sodium Nitrite?
In the world of food and health, few ingredients spark as much confusion and controversy - as sodium nitrate and sodium nitrite. Often lumped together in headlines and ingredient labels, these two chemical compounds are at the heart of an ongoing debate about food safety, preservation and health risks.
Are they dangerous additives? Are they essential preservatives? Or are they simply misunderstood?
Understanding the distinction between sodium nitrate and sodium nitrite is more than a matter of semantics - it’s crucial for consumers, food manufacturers and health professionals alike. Despite their similar names and overlapping uses, they behave differently in the body and serve distinct roles in food production.
Both compounds are commonly found in processed meats, such as bacon, ham, hot dogs and deli cuts. They play a key role in preserving color, preventing bacterial growth and extending shelf life. However, the way each compound functions - and how it is perceived in nutritional science - differs significantly.
In this blog post, we’ll break down the chemistry, clarify the confusion, and explain why knowing the difference matters. Whether you’re scanning a food label or developing a new formulation, getting this right can make all the difference.
What Are Sodium Nitrate and Sodium Nitrite?
When it comes to food safety and industrial chemistry, sodium nitrate and sodium nitrite are two compounds that often get grouped together, but they are chemically distinct and serve different purposes. Understanding their structures, origins and uses can help demystify their roles in everything from cured meats to fertilizer.
Basic Chemical Definitions
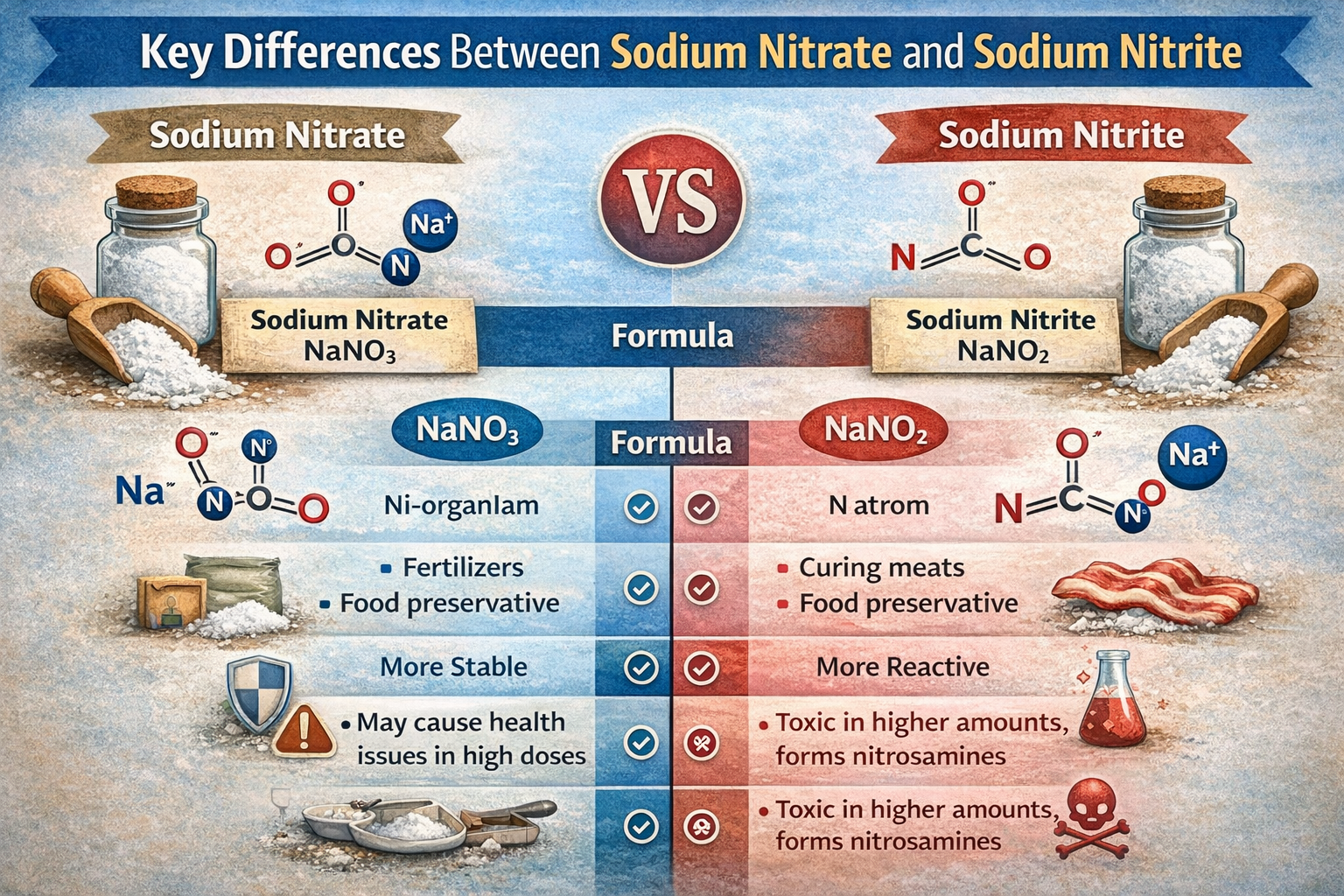
Sodium nitrate (NaNO₃) is a naturally occurring salt composed of sodium, nitrogen and oxygen. Its molecular structure includes one sodium ion (Na⁺) and one nitrate ion (NO₃⁻). In contrast, sodium nitrite (NaNO₂) contains one sodium ion and one nitrite ion (NO₂⁻), making it slightly more reactive and chemically different.
Sodium nitrate is often found in nature, particularly in arid regions such as Chile, where vast salt deposits have been historically mined. Sodium nitrite, however, is typically synthesized from nitrate through a chemical reduction process. While both can be produced synthetically for commercial use, their origins and reactivity differ significantly.
Common Uses in Food & Industry
In the food industry, both sodium nitrate and nitrite are valued for their preservative properties, especially in the curing of meats like bacon, ham and sausages. Nitrite, in particular, is responsible for giving cured meats their characteristic pink color and for preventing the growth of Clostridium botulinum, the bacterium that causes botulism.
Outside the kitchen, sodium nitrate has long been used in fertilizers due to its nitrogen content, and in explosives and pyrotechnics for its oxidizing properties. Sodium nitrite also finds industrial applications in dye production, corrosion inhibitors and pharmaceuticals.
Key Differences Between Sodium Nitrate and Sodium Nitrite
While sodium nitrate and sodium nitrite are chemically related, their behavior, function and health impact can differ significantly. One key point of confusion stems from the fact that nitrate can become nitrite under certain conditions, but this conversion is part of what defines their different roles.
Chemical Behavior and Conversion
Sodium nitrate (NaNO₃) is more stable than sodium nitrite (NaNO₂) and acts as a precursor in both food processing and the human body. In cured meats, nitrate is often slowly reduced to nitrite by bacterial action during the curing process. Similarly, in the digestive tract, enzymes and gut bacteria can convert ingested nitrate into nitrite.
This conversion is critical because nitrite is the compound that actively engages in preservation and antimicrobial activity. In essence, sodium nitrate’s role is indirect, it must become sodium nitrite to exert most of its effects in food preservation or physiological processes.
Function in Food Preservation
Sodium nitrite is the real workhorse in curing meats. It reacts with proteins in the meat to fix the pink color commonly associated with products like ham and bacon. Without it, cured meats would appear gray or brown, making them less appealing to consumers.
More importantly, sodium nitrite plays a vital safety role by inhibiting the growth of Clostridium botulinum, the bacterium responsible for botulism - a rare but deadly foodborne illness. This antimicrobial action is a key reason nitrite remains approved in regulated quantities despite ongoing health debates.
Nitrate, by contrast, is slower-acting and generally used when a longer curing process is involved. In many commercial applications, especially those requiring fast processing, nitrite is the compound of choice for reliable preservation and food safety.
Health Implications: Friend or Foe?
The use of sodium nitrate and sodium nitrite in food has sparked decades of debate among scientists, health advocates and consumers. While these compounds play a key role in preserving food and preventing dangerous bacteria, concerns about their long-term health effects continue to fuel public scrutiny.
Potential Health Risks
One of the main concerns surrounding sodium nitrite is its potential to form nitrosamines — chemical compounds that can develop when nitrite reacts with certain proteins at high temperatures, such as during frying or grilling. Some nitrosamines are carcinogenic in animal studies, leading to concerns about increased cancer risk in humans, particularly colorectal cancer.
Additionally, excessive consumption of sodium nitrate and nitrite may have cardiovascular implications. Some studies suggest that high levels could impact blood pressure regulation and overall vascular health. However, the evidence is mixed, and some research has even indicated possible cardiovascular benefits of dietary nitrates from vegetables, further complicating the narrative.
Regulatory Guidelines
To address these risks, food safety agencies worldwide have set strict limits on the use of nitrates and nitrites in food. The U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) both regulate their use, while the World Health Organization (WHO) has established acceptable daily intake (ADI) levels.
For sodium nitrite, the ADI is generally around 0.07 mg per kg of body weight per day, while sodium nitrate is slightly higher at 3.7 mg per kg. These limits aim to ensure that even regular consumers of processed meats remain within safe exposure levels, assuming a balanced diet.
Are Natural Alternatives Safer?
In response to consumer concerns, many food producers have turned to “natural” curing agents like celery powder, which is high in naturally occurring nitrates. Products made with these alternatives are often labeled as “uncured,” though the nitrate still converts into nitrite during processing, essentially performing the same function.
While natural options may appear safer, the chemistry remains largely the same. The key difference lies in perception and sourcing, not necessarily in health outcomes. Moderation, regardless of the source, remains the most important factor for consumers aiming to reduce risk.
Misconceptions and Label Confusion
The debate over sodium nitrate and nitrite has led to widespread confusion on supermarket shelves, especially when it comes to the difference between “cured” and “uncured” meat products. Marketing language often plays into consumer fears, but the truth behind these labels can be surprising.
“Uncured” vs “Cured” Products
Many products labeled as “uncured” still contain curing agents, just not the synthetic forms of sodium nitrate or nitrite. Instead, they often use natural sources like celery powder or beet juice, which are rich in naturally occurring nitrates. During processing, these nitrates are converted into nitrites, performing the same preservative and antimicrobial functions.
The “uncured” label, therefore, is more of a marketing term than a meaningful health distinction. It capitalizes on the perception that “natural” is automatically safer or better, even though the result is chemically similar.
Reading Food Labels Correctly
To truly understand what you’re consuming, it’s important to read ingredient lists carefully. Look for terms like “sodium nitrite,” “sodium nitrate,” or “cultured celery extract.” These all indicate the presence of nitrates or nitrites, whether synthetic or natural.
Understanding this language helps consumers make informed decisions rather than being misled by product labeling or buzzwords.
Are Sodium Nitrate and Nitrite Safe in Moderation?
Modern research suggests that sodium nitrate and sodium nitrite, when consumed within regulated limits, are generally safe for most people. While studies have linked excessive consumption of processed meats to certain health risks, these risks must be viewed in context. The presence of nitrates or nitrites alone doesn’t automatically make a food dangerous — dose, frequency, and overall diet quality matter.
For example, the occasional serving of bacon or deli meat is unlikely to pose a significant health risk for most individuals. In fact, many vegetables — such as spinach, celery, and beets — contain far higher levels of naturally occurring nitrates, which the body also converts into nitrites. The difference is that vegetables also contain antioxidants like vitamin C, which inhibit the formation of harmful nitrosamines.
It’s also important to weigh these risks against other lifestyle factors. Smoking, sedentary behavior, excessive alcohol consumption, and lack of fiber have all been shown to contribute more significantly to chronic diseases than moderate intake of processed meats.
The key takeaway? Moderation and balance are essential. Nitrates and nitrites play a functional role in food safety and preservation, and when consumed as part of a varied, nutrient-rich diet, they are not inherently harmful.
Conclusion
Sodium nitrate and sodium nitrite may sound similar, but their roles, behaviors and health implications are distinct. Nitrate serves as a precursor, while nitrite is the active agent in food preservation, helping to prevent spoilage and protect against harmful bacteria. Though concerns about health risks exist, especially when consumed in excess, regulatory limits and modern research support their safety in moderation.
Rather than fearing these compounds, consumers should aim to stay informed — reading labels, understanding context and making balanced dietary choices. Knowledge, not panic, is the best tool for navigating the “great salt confusion” with confidence.
DECACHEM is one of the main regional distributors of high-quality nitrates, supplying the food, industrial, and agricultural sectors with reliable and compliant materials.
FAQs
What’s worse: sodium nitrate or sodium nitrite?
Sodium nitrite is more reactive and directly involved in curing, so it tends to be more scrutinized. However, both are safe in regulated amounts.
Can I avoid nitrates and nitrites completely?
It’s difficult — these compounds are naturally found in many vegetables and water sources, not just processed foods.
Are naturally occurring nitrates better than added ones?
Chemically, they are nearly identical. The difference lies in the food context — vegetables offer protective nutrients like antioxidants.
Do vegetables contain nitrates?
Yes, leafy greens, beets, and celery are naturally high in nitrates and are generally considered healthy.
How can I tell if a product contains sodium nitrite?
Check the ingredient list for terms like "sodium nitrite," "sodium nitrate," or "cultured celery extract" (a natural source).