Tributyl Phosphate (TBP): A Key Extractant in Metal Separation and Nuclear Chemistry
Tributyl phosphate (TBP) is an organophosphate compound widely used in the chemical industry for its powerful solvent and extractant properties. TBP is a colorless, odorless liquid with moderate viscosity and low volatility. Its chemical structure consists of a central phosphorus atom bonded to three butoxy groups and a double-bonded oxygen, giving it both polar and nonpolar characteristics — ideal for dissolving or separating complex mixtures.
In industrial applications, TBP acts as an efficient chemical solvent and metal extractant, particularly in hydrometallurgy and nuclear fuel reprocessing. Its unique ability to selectively bind with certain metal ions makes it essential in the solvent extraction of rare earth elements and actinides such as uranium and plutonium. As a result, TBP plays a critical role in both metal separation technologies and nuclear chemistry, where precision and efficiency are paramount.
As a trusted supplier of high-quality chemical solutions,
DECACHEM is recognized as one of the main regional players in the distribution of Tributyl Phosphate (TBP). With a strong focus on quality, compliance and customer support, DECACHEM serves industries across Europe and the Middle East, supporting critical extraction and separation processes with reliable supply and technical expertise.
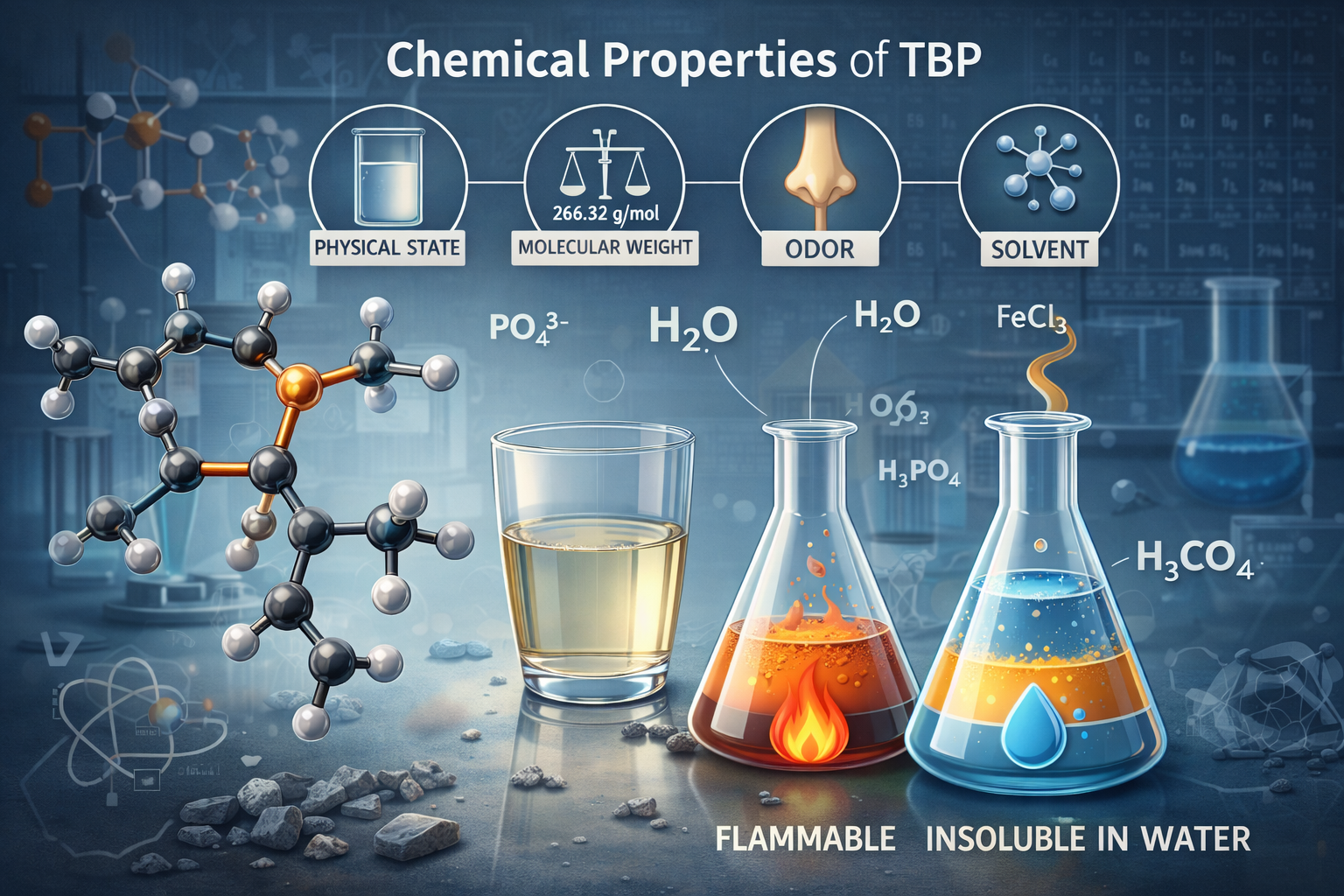
Chemical Properties of TBP
Tributyl phosphate exhibits a unique combination of physical and chemical properties that make it highly effective as a solvent and extractant in industrial and nuclear applications. It is a colorless, oily liquid with a mild odor and moderate viscosity.
In terms of chemical compatibility, TBP is generally stable and non-reactive under normal conditions. However, it may hydrolyze in the presence of strong acids or bases, releasing butanol and phosphoric acid derivatives. This makes it important to control pH levels during industrial use.
TBP demonstrates good thermal stability, but under extreme heat or prolonged radiation exposure — such as in nuclear reprocessing — it can undergo decomposition, forming acidic byproducts and potentially flammable gases. For this reason, TBP systems used in high-radiation environments must include safety measures for ventilation and temperature control.
These properties underline TBP’s versatility and reliability in both
metal separation and
nuclear fuel cycle applications.
TBP as an Extractant in Metal Separation
Role in Solvent Extraction Processes
Solvent extraction, also known as liquid-liquid extraction, is a widely used method for separating valuable metals from aqueous solutions using an organic solvent. Tributyl phosphate (TBP) plays a key role in this process due to its ability to form stable complexes with specific metal ions. TBP is particularly selective toward rare earth elements, actinides and certain transition metals, making it ideal for high-precision separation tasks.
When dissolved in a suitable organic diluent (e.g., kerosene), TBP extracts metal ions from an aqueous solution by forming organophosphorus-metal complexes. Its chemical structure allows it to interact effectively with metals in high oxidation states, facilitating their transfer into the organic phase. This selectivity and efficiency make TBP a preferred extractant in metallurgical and nuclear applications.
Industrial Applications in Metal Recovery
TBP enables clean separation and purification of these radioactive metals for recycling or safe disposal. In addition, TBP is used for the separation of thorium, an important element in advanced nuclear fuel technologies, and in the recovery of lanthanides such as neodymium, cerium and lanthanum from mixed ores or industrial waste streams. TBP also facilitates the isolation of actinides, helping in the production of high-purity compounds for scientific or military purposes.
Thanks to its high extraction efficiency, metal selectivity and ability to operate under demanding conditions, TBP remains a cornerstone in metal extraction technologies, especially in nuclear and rare earth industries.
TBP in Nuclear Chemistry
PUREX Process and TBP
One of the most critical applications of Tributyl Phosphate is in the PUREX process (Plutonium Uranium Redox Extraction), a cornerstone technology in nuclear fuel reprocessing. Developed in the mid-20th century, the PUREX process is used to separate and purify plutonium and uranium from spent nuclear fuel, allowing for their reuse or safe disposal.
In this process, TBP is diluted with an organic solvent (typically kerosene) and brought into contact with an aqueous solution containing dissolved nuclear fuel. TBP selectively extracts uranium and plutonium into the organic phase by forming stable coordination complexes. These metals are then separated from fission products and other actinides, purified and later stripped from the organic phase for reuse.
TBP’s role as a key extractant in the PUREX process lies in its high selectivity for actinides, operational stability, and ability to be recycled for multiple extraction cycles without significant degradation.
Advantages of Nuclear Reprocessing
The use of TBP in nuclear chemistry offers several significant advantages:
- High Selectivity: TBP shows a strong preference for extracting actinide ions over other fission products, allowing for precise separation of
plutonium and uranium.
- Radiation Resistance: Although TBP can degrade under high radiation, it remains relatively stable during multiple cycles, especially when protected by process controls and antioxidant additives.
- Chemical Recyclability: TBP can be regenerated and reused, reducing chemical waste and enhancing the sustainability of nuclear reprocessing operations.
Given its performance, TBP remains a standard extractant in nuclear fuel reprocessing worldwide.
Safety, Toxicity and Handling Considerations
While Tributyl Phosphate (TBP) is widely used in industrial and nuclear applications, it requires careful handling due to its toxicity and potential environmental impact. According to the TBP’s safety data sheet (SDS), prolonged or repeated exposure can lead to skin and eye irritation, as well as respiratory issues if inhaled in aerosol or vapor form. TBP is classified as harmful if swallowed and may affect the liver and kidneys with long-term exposure.
From an environmental perspective, TBP is not readily biodegradable and may pose risks to aquatic ecosystems if released in large quantities. It is considered hazardous to aquatic life, so measures must be taken to prevent spills or uncontrolled discharges into water bodies.
To ensure safe handling, TBP should be stored in tightly sealed containers, in a cool, dry, and well-ventilated area, away from sources of heat or ignition. Use of personal protective equipment (PPE) - including gloves, goggles, and protective clothing - is recommended during handling. For disposal, TBP must be treated as hazardous chemical waste and should be managed according to local environmental regulations, typically via high-temperature incineration.
DECACHEM always provides technical documentation and safety data sheets to ensure responsible and compliant use across industries.
Alternatives and Innovations
While Tributyl Phosphate remains a gold standard in metal extraction and nuclear reprocessing, growing environmental and safety concerns have driven research into TBP alternatives and more eco-friendly extractants. Emerging options include ionic liquids, supercritical CO₂-based solvents, and green solvents derived from bio-based materials. These alternatives aim to reduce toxicity, enhance biodegradability, and minimize environmental impact.
In the realm of green chemistry, innovations focus on designing extraction systems that are both effective and sustainable. For example, researchers are developing phosphorus-free ligands and functionalized polymers that offer comparable selectivity with improved safety profiles.
Looking ahead, the future of extractant research lies in balancing performance with environmental responsibility. Advances in computational modeling and nanotechnology may also pave the way for tailor-made solvents that meet the evolving needs of metal and nuclear industries.
Conclusion
Tributyl Phosphate remains a cornerstone chemical in both metal separation and nuclear fuel reprocessing, thanks to its unique selectivity, chemical stability and versatility as an extractant. From recovering valuable rare earth elements to enabling the PUREX process in nuclear chemistry, TBP plays a vital role in high-precision industries.
As the demand for cleaner, safer technologies grows, TBP continues to evolve alongside research into greener alternatives. With ongoing innovations and responsible handling, TBP will likely remain essential in industrial and nuclear applications for years to come.
DECACHEM proudly supports this progress as a trusted regional supplier of high-purity TBP.
Frequently Asked Questions (FAQ)
Q1. What makes TBP ideal for nuclear reprocessing?
TBP’s high selectivity for uranium and plutonium ions, combined with its chemical stability and ability to be recycled, makes it essential in nuclear fuel reprocessing processes like PUREX. Its efficiency ensures precise separation of valuable actinides from spent fuel.
Q2. Is TBP harmful to humans or the environment?
While TBP is generally safe when handled properly, it can cause skin, eye, and respiratory irritation. It is toxic if ingested and poses risks to aquatic life if released untreated. Following the safety data sheet (SDS) guidelines is critical.
Q3. What are safer alternatives to TBP in solvent extraction?
Emerging
green solvents such as ionic liquids and bio-based extractants offer lower toxicity and better biodegradability. These
TBP alternatives are gaining attention for environmentally friendly metal separation.
Q4. Can TBP be recycled after use in industrial processes?
Yes, TBP is chemically recyclable and can be purified and reused multiple times, reducing waste and cost in industrial and nuclear applications.
Q5. How does TBP compare with other extractants in terms of selectivity?
TBP is known for its high selectivity toward actinides and rare earth metals, often outperforming many other extractants in nuclear chemistry. However, new extractants are being developed to improve selectivity and environmental safety.