Phosphates in Flame Retardant Systems: Mechanisms and Benefits
Introduction to Flame Retardant Systems
Why Flame Retardants Are Critical in Modern Materials
From consumer electronics and transportation to construction and textiles, modern materials are expected to be lightweight, durable, and high-performing, yet also safe in fire scenarios. Polymers and polymer-based composites, while versatile and economical, are inherently combustible. When exposed to heat, they undergo thermal degradation of polymers, releasing flammable gases that can ignite and sustain flames. Without protection, a small ignition source can escalate into rapid flame spread, structural failure, and the release of toxic smoke.
Flame retardants are additives or reactive components designed to interrupt this process. They either slow ignition, reduce flame propagation, or suppress heat and smoke release. The result is more time for evacuation, reduced fire damage, and improved compliance with increasingly strict fire safety materials standards across industries.
Growing Demand for Halogen-Free Flame Retardants
Historically, brominated and chlorinated flame retardants dominated the market due to their high efficiency in the gas phase. However, concerns over persistence, bioaccumulation, and toxic byproducts (such as corrosive hydrogen halides and dense smoke) have accelerated the shift toward halogen-free flame retardants. Regulatory frameworks like REACH, RoHS, and various eco-labels now favor alternatives with improved environmental and health profiles.
This shift has positioned phosphates - a key class of phosphorus-based flame retardants - as front-runners. Phosphate
flame retardants offer multi-mechanism protection, lower smoke toxicity, and strong performance in a wide range of polymers. Their versatility and regulatory alignment make them central to next-generation fire protection strategies.
What Are Phosphates in Flame Retardant Systems?
Chemical Structure and Classification of Phosphate Flame Retardants
Phosphate flame retardants are compounds containing phosphorus in the form of phosphate, phosphonate, or phosphinate groups. Their effectiveness stems from the chemistry of phosphorus, which can act in both the condensed phase (solid polymer) and the gas phase (flame zone).
They are commonly classified as:
Organic phosphates – Phosphorus bonded to carbon-based groups (e.g., aryl or alkyl phosphates). These are often liquid or low-melting and can act as plasticizers.
Inorganic phosphates – Salts or polymeric forms such as ammonium polyphosphate. These are typically solid and function primarily in the condensed phase.
This flexibility allows formulators to tailor performance, processing behavior, and durability for specific applications.
Common Phosphate Compounds Used in Industry
Ammonium Polyphosphate (APP)
APP is one of the most widely used phosphate flame retardants, especially in intumescent flame retardant systems. It decomposes upon heating to release phosphoric acid, which promotes char formation in polymers and creates a protective barrier.
Organophosphates
These include compounds like triphenyl phosphate (TPP) and resorcinol bis(diphenyl phosphate) (RDP). They are commonly used in engineering plastics, polyurethanes, and coatings. Organophosphates can act in both the condensed and gas phases and often provide good compatibility with polymer matrices.
Phosphonates and Phosphinates
These are used in high-performance thermoplastics such as polyamides and polyesters. Aluminum diethyl phosphinate (AlPi), for example, is valued for its thermal stability and effectiveness in demanding applications like electronics housings.
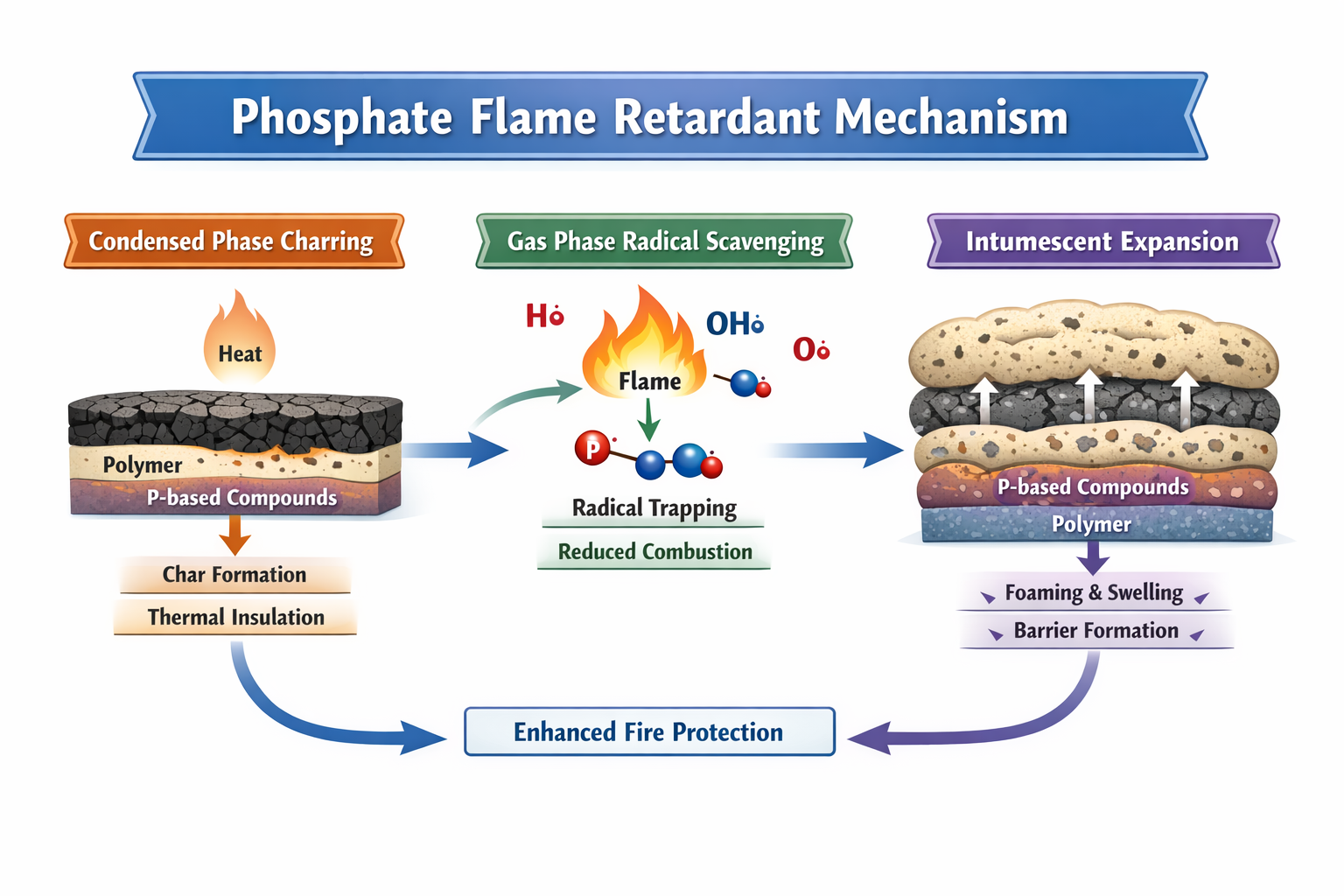
Mechanisms of Flame Retardancy in Phosphate Systems
Phosphate-based systems work through multiple, complementary flame-retardant mechanisms. This multi-action behavior is one of their greatest strengths.
Condensed Phase Mechanism
In the condensed phase, phosphates promote the formation of a stable, carbonaceous char layer on the polymer surface. When exposed to heat:
- Phosphate compounds decompose to form phosphoric acid derivatives.
- These acids catalyze dehydration reactions in the polymer.
- The polymer chains crosslink and aromatize, leading to char formation in polymers.
This char acts as a physical barrier that:
- Insulates the underlying material from heat.
- Reduces the release of flammable volatiles.
- Slows further thermal degradation of polymers.
- The result is a reduced heat release rate and delayed flame spread.
Gas Phase Flame Inhibition
Some phosphorus-based flame retardants also act in the gas phase. When the polymer decomposes, volatile phosphorus species enter the flame zone. There they:
- Interact with high-energy radicals such as H· and OH·.
- Disrupt the radical chain reactions that sustain combustion.
- Lower the flame temperature and slow oxidation.
This gas-phase flame inhibition complements the condensed phase barrier, making phosphate systems effective even at relatively low loadings.
Intumescent Flame Retardant Action
Intumescent systems are a specialized category where phosphates play a central role. A typical intumescent flame retardant system includes:
- Acid source (e.g., APP)
- Carbon source (e.g., pentaerythritol)
- Blowing agent (e.g., melamine)
Upon heating, the acid source generates phosphoric acid, which reacts with the carbon source to form char. Simultaneously, the blowing agent releases non-flammable gases that cause the char to swell - creating a thick, foamed, insulating layer. This “swelling char” dramatically reduces heat transfer and oxygen diffusion to the polymer surface.
Benefits of Using Phosphates as Flame Retardants
High Flame Retardant Efficiency
Phosphates provide strong fire performance across many polymer systems. Their ability to act in both phases (solid and gas) means that smaller amounts can achieve required fire classifications such as UL 94 V-0, limiting oxygen index (LOI), or cone calorimeter targets.
Compatibility with Various Polymer Matrices
Phosphate flame retardants are compatible with:
- Polyolefins (PP, PE)
- Engineering plastics (PA, PET, PBT, PC blends)
- Polyurethanes and epoxy resins
- Liquid organophosphates can also function as plasticizers, improving flexibility and processing.
Halogen-Free and Environmentally Favorable Profile
As halogen-free flame retardants, phosphates avoid the formation of corrosive and toxic halogenated gases during combustion. Many phosphate systems show improved environmental behavior, reduced persistence, and better alignment with modern chemical management programs.
Reduced Smoke and Toxic Gas Emissions
Compared with brominated systems, phosphate flame retardants typically generate less dense smoke and fewer toxic combustion byproducts. This improves visibility and survivability during fires, an increasingly important factor in building and transportation safety.
Comparative note: Brominated flame retardants excel in gas-phase inhibition but can produce heavy smoke and toxic halogen acids. Phosphate systems, by contrast, balance condensed phase protection with cleaner combustion profiles.
Applications of Phosphate Flame Retardant Systems
Plastics and Polymer Composites
Phosphate systems are widely used in:
- Automotive interior components
- Consumer electronics housings
- Appliance parts
They provide fire protection without sacrificing mechanical strength or surface finish.
Textiles and Coatings
In textiles, phosphate-based finishes impart durable flame retardancy to fibers used in protective clothing, upholstery, and curtains. In coatings, intumescent phosphate systems protect steel structures by forming an insulating char during a fire.
Electrical & Electronic Components
Printed circuit boards, connectors, and enclosures rely on phosphorus-based flame retardants for compliance with strict electrical safety standards. Aluminum phosphinates and organophosphates are especially important here due to their thermal stability.
Construction and Building Materials
Insulation foams, wall panels, and sealants benefit from phosphate systems that reduce flame spread and smoke generation, supporting modern building codes and green building certifications.
Regulatory and Sustainability Considerations
Compliance with REACH, RoHS and Global Fire Safety Standards
Phosphate flame retardants are widely accepted under the REACH and RoHS frameworks when properly formulated. They support compliance with European, North American, and Asian fire safety standards without relying on restricted halogens.
Role of Phosphates in Sustainable Fire Protection Solutions
Sustainability trends favor:
- Reactive phosphate systems that chemically bond to polymers, reducing migration.
- Bio-based phosphates derived from renewable feedstocks.
- Lower-toxicity, recyclable formulations.
- These innovations position phosphates as key contributors to sustainable fire-safe materials.
Challenges and Limitations of Phosphate Flame Retardants
Moisture Sensitivity and Migration Issues
Some inorganic phosphates, like APP, can be hygroscopic. Without proper encapsulation, they may absorb moisture or migrate to the surface over time.
Processing and Thermal Stability Concerns
Certain organophosphates can volatilize or decompose at high processing temperatures. Selecting thermally stable grades is essential for engineering plastics.
Cost and Formulation Complexity
High-performance phosphate systems may cost more and require careful formulation with synergists (nitrogen, silicon) to optimize performance.
Future Trends in Phosphate-Based Flame Retardant Systems
Nano-Enhanced Phosphate Flame Retardants
Nanostructured additives improve dispersion and char integrity, enhancing barrier properties at lower loadings.
Synergistic Systems with Nitrogen and Silicon
Combining phosphorus with nitrogen (melamine derivatives) and silicon (siloxanes) yields stronger, more stable chars and better smoke suppression.
Innovations in Reactive and Bio-Based Phosphates
Reactive phosphates that bond into polymer chains and bio-based phosphorus sources are driving the next wave of sustainable fire safety materials.
Frequently Asked Questions (FAQs)
How do phosphate flame retardants differ from halogenated ones?
Phosphates are halogen-free flame retardants that rely on char formation and radical scavenging, while halogenated systems mainly inhibit combustion in the gas phase. Phosphates typically produce less toxic smoke and are more environmentally acceptable.
Are phosphate flame retardants environmentally safe?
Many phosphate systems show improved environmental profiles compared to older halogenated products. Ongoing innovation focuses on low-toxicity, non-migrating, and bio-based solutions.
Which polymers benefit most from phosphate flame retardants?
Engineering plastics (PA, PET, PC blends), polyurethanes, epoxies, and polyolefins all benefit from phosphate-based systems, especially where both fire safety and low smoke are required.
Do phosphate systems affect mechanical properties?
When properly formulated, phosphate flame retardants can maintain or even improve flexibility and toughness. However, high loadings may require reinforcement or synergists to preserve strength.
Conclusion
Phosphates have become essential players in modern flame-retardant technology. Through a combination of condensed phase flame retardants, gas phase flame inhibition, and intumescent flame retardant systems, they deliver reliable fire protection while aligning with environmental and regulatory demands. Their ability to promote char formation in polymers, reduce smoke, and support halogen-free strategies makes them ideal for next-generation fire safety materials. As innovation continues, through nano-enhancement, synergistic formulations, and bio-based chemistry, phosphates will remain central to the development of safer, smarter, and more sustainable materials.
















