Mining Chemicals: Types and Applications (Part 1)
Mining chemicals are indispensable to the modern mining industry, playing a pivotal role in the efficient extraction and processing of ores. These specialized chemicals enhance separation techniques, improve recovery rates, and ensure the economic viability of mining operations. From frothers and collectors to depressants and flocculants, each chemical is tailored to optimize specific stages of the mineral extraction process.
In an era of increasing demand for minerals and metals, mining chemicals are also crucial in promoting sustainability. Advanced formulations minimize waste and environmental impact while maximizing resource efficiency, aligning with global goals for greener operations.
Among the leaders in this field is Decachem, a trusted provider of high-quality solutions for the mining sector. Decachem’s innovative products exemplify the industry's reliance on specialized chemicals to meet the challenges of modern mining. With a focus on efficiency and environmental responsibility, Decachem remains one of the best in business.
What Are Mining Chemicals?
Mining chemicals are specialized compounds designed to optimize and support the various stages of the mining lifecycle, from ore extraction to mineral processing and waste management. These
industrial chemicals for mining play a critical role in increasing efficiency, enhancing yield, and reducing environmental impact across the mining value chain.
At the heart of their application lies their ability to manipulate the physical and chemical properties of minerals, making separation and extraction more effective. Reagent chemicals, such as collectors, frothers, depressants and flocculants, are used to refine the processes of flotation, leaching and solid-liquid separation. Their precision ensures that valuable minerals are extracted efficiently, while unwanted impurities are minimized or removed altogether.
Enhancing Yield and Reducing Waste
One of the most significant benefits of using
mineral processing chemicals is their ability to maximize resource recovery. By fine-tuning chemical reactions during extraction, mining companies can improve the yield of high-value minerals, such as gold, copper and rare earth elements, from even low-grade ores. This increases the economic viability of mining operations while simultaneously reducing the volume of waste generated.
In addition, these chemicals are indispensable for minimizing water and energy consumption, both critical resources in mining. Advanced chemical formulations also help reduce the release of harmful by-products, aligning mining practices with stricter environmental regulations and sustainability goals.
Indispensable Across Sectors
The importance of mining chemicals spans multiple sectors:
● Gold Mining: Specialized reagents aid in cyanide leaching and froth flotation, essential for gold recovery.
● Coal Mining: Flocculants and coagulants are critical in dewatering and fine coal recovery.
● Rare Earth Mining: Collectors and extractants are used to separate rare earth elements crucial for advanced technologies.
● Base Metals (Copper, Zinc, Nickel): Tailored reagent chemicals improve flotation performance and impurity removal.
As global demand for minerals continues to grow, the reliance on these chemicals is set to increase. Companies like Decachem, a leader in industrial chemicals for mining, deliver innovative and sustainable solutions that address both efficiency and environmental challenges. By optimizing chemical use, Decachem helps mining operations meet the evolving needs of the industry while ensuring responsible resource management.
Mining chemicals are not just tools of the trade - they are the catalysts driving the future of a more efficient and sustainable mining industry.
Types of Mining Chemicals
Mining chemicals are specialized compounds used to optimize various stages of the mining process, enhancing efficiency, yield, and sustainability. These chemical reagents for mining are tailored for specific applications, from mineral separation to waste management, making them indispensable in modern mining operations.
Flotation Reagents
Flotation reagents are critical in separating valuable minerals from ores, particularly in sulfide ore flotation. These chemicals manipulate the surface properties of minerals, enabling selective separation.
● Frothers: Stabilize froth in the flotation cell by reducing water surface tension, creating a foam layer that carries valuable minerals to the surface.
○ Examples: Methyl isobutyl carbinol (MIBC), and pine oil.
● Collectors: Make mineral surfaces hydrophobic, ensuring attachment to air bubbles for flotation.
○ Examples: Xanthates (e.g., sodium ethyl xanthate), dithiophosphates.
● Depressants: Prevent unwanted minerals from attaching to the froth, improving purity.
○ Examples: Sodium cyanide (for pyrite suppression), and starch (for gangue minerals).
Flotation agents are essential for recovering gold, copper, lead, and other valuable minerals.
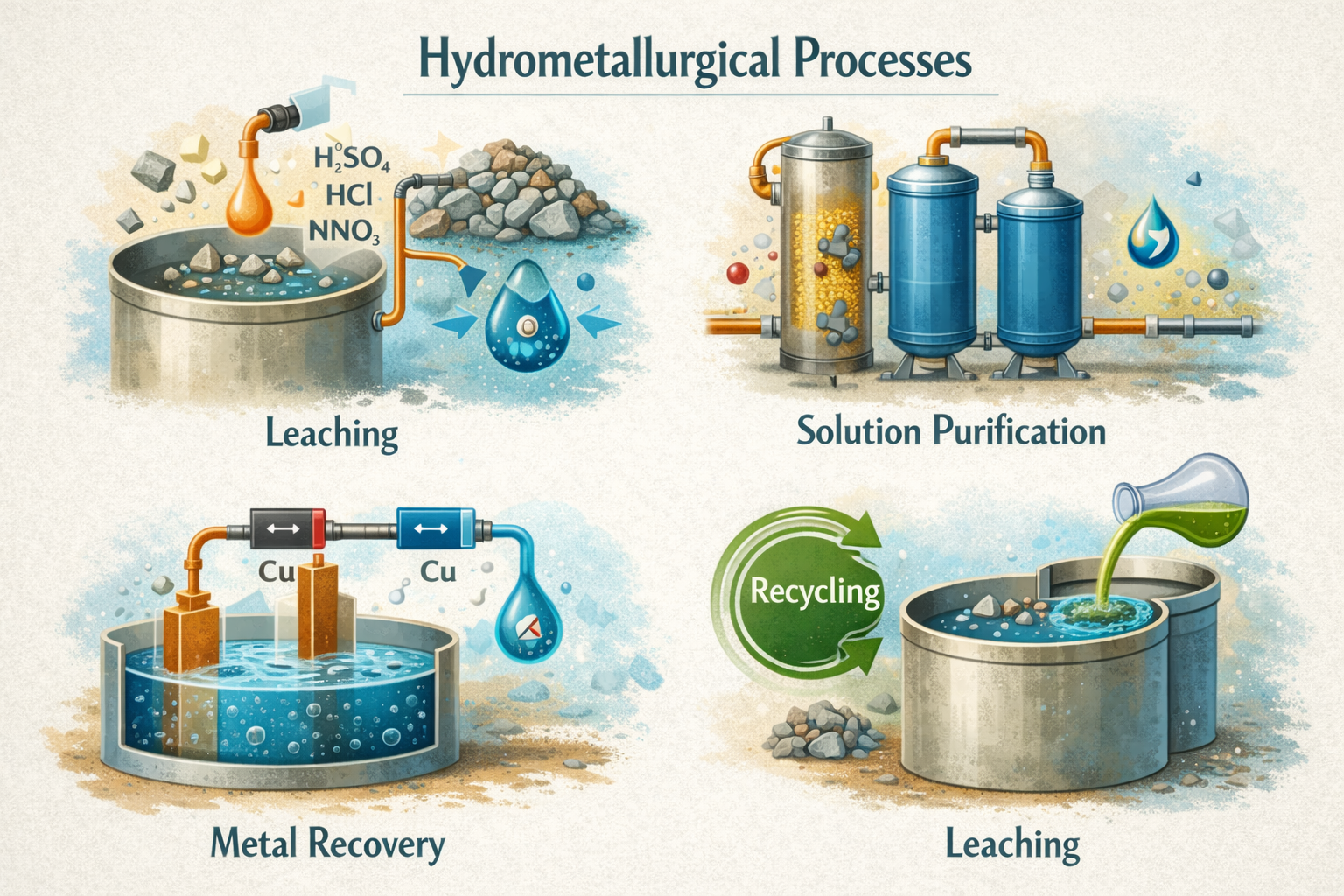
Extractants and Leaching Agents
Used in hydrometallurgical processes, extractants and leaching agents dissolve and separate metals like gold and copper from ores.
● Cyanide: Widely used for extracting gold through cyanidation, where gold dissolves in a cyanide solution.
● Thiourea: An alternative to cyanide, offering less environmental impact for gold extraction.
● Organic Solvents: Extract specific metals during solvent extraction processes in copper refining.
These reagents help recover metals efficiently while striving to minimize environmental harm.
Grinding Aids
Grinding aids improve milling efficiency by reducing energy consumption and preventing over-grinding. These chemicals enhance ore breakage and particle size reduction during milling.
● Examples: Glycol-based chemicals and polycarboxylate ethers.
By improving throughput, grinding aids are vital in reducing operational costs and optimizing ore preparation.
Dewatering Aids
Dewatering aids assist in water recovery from mineral concentrates and tailings, crucial for managing water resources and waste.
● Flocculants: Aggregate fine particles into larger clumps for easier water removal.
○ Example: Polyacrylamides.
● Coagulants: Neutralize particle charges to promote aggregation.
○ Example: Ferric chloride.
These dewatering solutions are essential for efficient tailings management and sustainable water use in mining operations.
By combining these specialized chemicals, the mining industry achieves greater resource efficiency and adheres to environmental standards, ensuring a sustainable future.
Applications of Mining Chemicals in the Industry
Mining chemicals play a critical role in modern mining operations, contributing to efficiency, sustainability, and safety. Their applications span multiple stages of the mining process, from ore beneficiation to waste management and hazard reduction, making them indispensable for industrial-scale operations.
Ore Processing and Beneficiation
In the initial stages of mining, beneficiation chemicals are used to extract valuable minerals from raw ore. These chemicals aid in crushing, grinding, and separation processes, enhancing mineral recovery and improving operational efficiency.
● Flotation reagents (frothers, collectors, depressants) are essential for separating valuable minerals from gangue.
● Grinding aids improve milling efficiency, reducing energy consumption during ore size reduction.
● Flocculants and dispersants optimize solid-liquid separation during concentration and filtration.
These chemicals ensure high recovery rates and allow mining operations to process even low-grade ores efficiently.
Waste Management
Effective waste management is a cornerstone of sustainable mining, and environmental solutions for mining rely heavily on specialized chemicals to treat and manage waste products.
● pH Adjusters: Lime and caustic soda regulate pH levels in tailings ponds to neutralize acidic waste.
● Neutralizing Agents: Used to detoxify harmful chemicals like cyanide in waste streams.
● Coagulants and flocculants: Aid in water recovery and reduce the environmental footprint of tailings.
These chemicals help mining companies comply with environmental regulations and minimize the ecological impact of their operations.
Safety and Hazard Reduction
Mining environments often involve handling hazardous materials. Mining safety measures include the use of chemicals designed to reduce risks and protect workers.
● Dust suppressants: Prevent airborne particulate matter during drilling and transportation.
● Inhibitors and stabilizers: Mitigate the risks of spontaneous combustion in coal mines.
● Chemical encapsulants: Safely contain hazardous by-products.
By integrating these solutions, mining operations reduce the risks of chemical exposure, enhancing workplace safety while maintaining regulatory compliance.
From optimizing ore beneficiation to ensuring safe and sustainable waste disposal, mining chemicals underpin the industry’s ability to balance efficiency with environmental responsibility.
















