10 Surprising Facts About Phosphates You (probably) Didn’t Know
When was the last time you thought about phosphates? Chances are, they rarely cross your mind. But these remarkable compounds play a vital role in nearly every aspect of daily life. From the food we eat to the crops that feed the world, from cleaning products to industrial processes, phosphates are the quiet workhorses behind the scenes, ensuring efficiency, productivity and sustainability.
Despite their importance, phosphates often go unnoticed, overshadowed by the products and industries they support. But there’s much more to these compounds than meets the eye.
As one of the main players in the business of phosphate trading and distribution,
Decachem
is committed to bringing the best of these indispensable compounds to industries worldwide. In this blog post, we’re uncovering
10 surprising facts about phosphates that highlight their versatility, importance and fascinating history. Whether you’re a chemistry enthusiast or simply curious about the hidden forces shaping our world, these insights will give you a newfound appreciation for this indispensable family of compounds. Let’s dive in!
1. Phosphates Are Integral to DNA and RNA Structures
Phosphates are fundamental to the structure of DNA and RNA, forming the sugar-phosphate backbone that provides stability and support to these essential molecules. In both DNA and RNA, phosphate groups link the 3' carbon atom of one sugar molecule (deoxyribose in DNA, ribose in RNA) to the 5' carbon atom of the next, creating a strong covalent bond known as a phosphodiester bond. This linkage not only maintains the integrity of the nucleotide chain but also ensures proper orientation for genetic encoding. Without phosphates, the structural framework of DNA and RNA would collapse, making genetic information storage and transfer impossible.
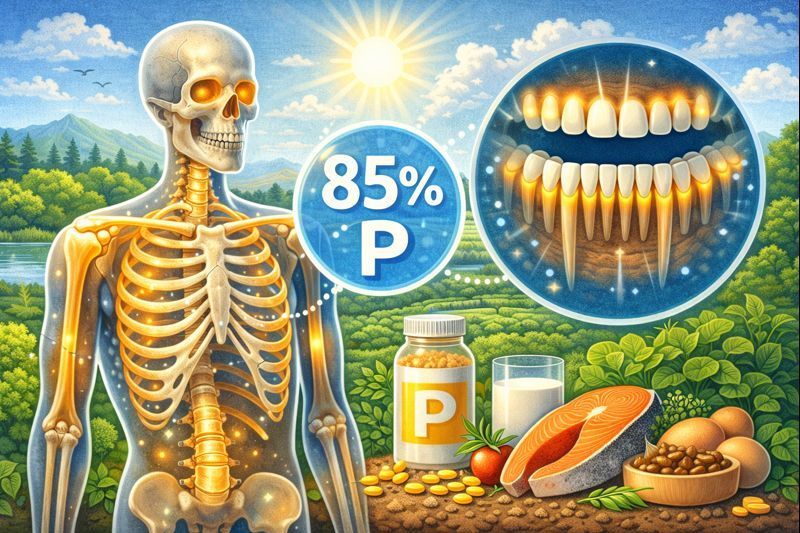
2. The Human Body Contains Significant Amounts of Phosphorus
Approximately
85% of the body’s phosphorus is stored in bones and teeth, where it combines with calcium to form hydroxyapatite, a mineral that provides strength and rigidity. This reservoir of
phosphorus is vital not only for skeletal health but also as a readily accessible supply for other physiological needs. The remaining 15% of phosphorus is distributed throughout the body in cells and tissues. Here, it plays critical roles in energy transfer (as ATP), cell signaling, and the formation of DNA and RNA. This balance ensures phosphorus supports both structural and metabolic functions, making it essential for overall health and vitality.
3. Phosphates Play a Key Role in Energy Transfer Within Cells
Phosphates are integral to adenosine triphosphate (ATP), often called the "energy currency" of cells. ATP consists of adenine, ribose (a sugar), and three phosphate groups linked by high-energy bonds. These phosphate bonds store and release energy essential for cellular processes.
When cells require energy, ATP undergoes hydrolysis, breaking the bond between the second and third phosphate groups to form adenosine diphosphate (ADP) and an inorganic phosphate (Pi). This reaction releases energy that powers vital activities like muscle contraction, protein synthesis and active transport.
Phosphates in ATP also play a regulatory role in signaling pathways, enabling enzymes and proteins to function efficiently. Without phosphates, ATP could not perform its critical role in maintaining life’s energy demands.
4. Phosphates Are Essential Components in Fertilizers
Phosphates are vital nutrients for plant growth, playing a crucial role in several key processes. They are a primary component of
DNA, RNA and
ATP, which are essential for cell division, energy transfer and overall plant metabolism.
Phosphorus, in the form of phosphate ions, is absorbed by plants from the soil and used to produce
phospholipids, which are integral to cell membranes. Phosphates also promote
root development,
flowering and
fruiting, enhancing plant productivity. A deficiency in phosphate can lead to stunted growth, poor root development, and reduced yield. Therefore, ensuring adequate phosphate availability in the soil is critical for healthy plant development and agriculture.
5. Phosphates Are Used in Processed Foods as Additives
Phosphates are commonly used in food products to enhance texture, moisture retention, and overall product quality. In processed meats, like sausages and deli meats, phosphates help retain water during processing, preventing the loss of moisture during cooking and extending shelf life. This improves the product’s juiciness and mouthfeel. In baked goods, phosphates act as leavening agents, aiding in the rise and texture of bread and cakes by reacting with acidic ingredients to release carbon dioxide. They also stabilize dairy products, such as cheese and milk, by preventing calcium from precipitating, ensuring smoothness and consistency. Additionally, phosphates are used in canned vegetables and fruits to maintain firmness and texture during storage. By enhancing moisture retention and texture, phosphates contribute to the quality and consumer appeal of a wide variety of food products.
6. Phosphates Are Key Ingredients in Detergents
Phosphates in detergents play a crucial role in softening water and enhancing cleaning efficiency. Hard water contains high levels of calcium and magnesium ions, which can interfere with the cleaning action of detergents by binding with soap molecules and forming insoluble compounds, reducing their effectiveness. Phosphates act as water softeners by binding to these calcium and magnesium ions, preventing them from interfering with the cleaning process. This helps detergents form a more effective lather and ensures better stain removal. Additionally, phosphates help maintain the detergent’s pH level, optimizing its performance. Although their use in household detergents is being phased out in some regions due to environmental concerns, phosphates continue to be important in industrial cleaning products for their ability to improve efficiency and reduce mineral build-up.
7. Phosphates Are Involved in Environmental Concerns
Phosphates can contribute significantly to
water pollution and
eutrophication when they enter aquatic ecosystems in excessive amounts. Eutrophication occurs when water bodies receive high concentrations of nutrients, particularly phosphates, often from agricultural runoff, sewage and detergents. These excess phosphates act as fertilizers, stimulating the rapid growth of algae and aquatic plants. While this may initially seem beneficial, it leads to
algal blooms that deplete oxygen levels in the water, a process known as hypoxia. As algae die and decompose, the oxygen depletion becomes more severe, creating "dead zones" where most aquatic life cannot survive. The imbalance in the ecosystem also reduces biodiversity and disrupts the natural food chain. Addressing phosphate pollution through better agricultural practices, wastewater treatment and reduced phosphate use in detergents is crucial to preventing and mitigating eutrophication.
8. Phosphates Are Used in Flame Retardants
Phosphate compounds are widely used in materials to
reduce flammability and enhance fire resistance. In
flame-retardant coatings and
plastics, phosphates, such as
ammonium polyphosphate, form a protective char layer when exposed to heat or flames. This layer insulates the material, preventing further combustion and reducing the spread of fire. Phosphates also help in the formation of
non-combustible gases, such as phosphoric acid vapors, which act as flame inhibitors by disrupting the chemical reactions necessary for combustion. These compounds are commonly used in building materials, textiles, electrical cables, and automotive components to improve safety and meet fire regulations. Their ability to withstand high temperatures makes phosphate-based flame retardants an essential part of fire-resistant material development.
9. Phosphates Are Present in Some Soft Drinks
Phosphoric acid is commonly used in beverages, particularly colas, to provide a distinctive tangy flavor and enhance overall taste. Its sharp, acidic profile adds a refreshing zest that balances the sweetness of sugar or artificial sweeteners. Phosphoric acid also contributes to the refreshing sensation in sodas by stimulating the taste buds with its slight bitterness, which helps to create a more complex and enjoyable flavor profile. Additionally, it acts as a preservative, helping to extend the shelf life of beverages by lowering the pH and inhibiting microbial growth. While phosphoric acid is primarily used for flavor enhancement, it also helps to stabilize carbonation in fizzy drinks, maintaining the effervescence and sensory experience of sparkling sodas. However, excessive consumption has been linked to potential health concerns, prompting some to seek alternatives in beverage formulations.
10. Phosphates Have a Role in Medical Applications
Phosphate compounds are used in medicine, particularly in laxatives and dietary supplements, due to their ability to influence bodily functions. In laxatives, compounds like sodium phosphate are effective in relieving constipation by drawing water into the intestines, softening stool, and stimulating bowel movements. This promotes quicker and more comfortable elimination. Phosphate-based dietary supplements are also used to address phosphorus deficiencies, particularly in individuals with kidney disorders or those on restricted diets. Phosphates in these supplements help support bone health, cellular energy production and muscle function. They also play a role in maintaining proper acid-base balance in the body. However, phosphate-based medications should be used carefully under medical supervision, as excessive intake can lead to electrolyte imbalances or kidney damage, particularly in individuals with pre-existing kidney conditions.
In conclusion, phosphates are far more fascinating and versatile than we often realize. From being the backbone of
DNA and RNA, crucial for life’s genetic instructions, to providing
energy through ATP, they are essential for cellular processes. Phosphates are also key players in
plant growth, enhancing
soil fertility and promoting
root development, which ultimately supports global food production. In the food industry, they improve
texture,
moisture retention, and
taste, ensuring the quality of products we consume daily. Additionally, phosphates are used in
detergents to soften water, in
flame retardants to improve fire resistance, and in
medicine to treat constipation and support bone health. These surprising applications underscore the
incredible importance of phosphates in various facets of life: agriculture, industry, health and beyond. As we continue to innovate, phosphates remain essential to the progress and functioning of countless processes that sustain modern life.
















