Nitrates and Fireworks: The Chemistry of Celebration
Introduction: Lighting Up the Sky With Chemistry
From New Year’s Eve to national holidays, fireworks have long dazzled the skies in celebrations around the world. Whether it's a quiet sparkler or a thunderous aerial burst, these light shows are more than just entertainment, they're rooted in centuries of cultural tradition and scientific innovation.
At the heart of every spectacular firework display lies chemistry. A firework is essentially a controlled explosion packed into a shell, designed to produce color, sound and light. The beauty we see overhead begins with the precise combination of oxidizers, fuels, binders and color-producing metal salts.
Among these components, nitrates play a starring role. Compounds like potassium nitrate, sodium nitrate, and barium nitrate act as powerful oxidizers - substances that provide oxygen to fuel the rapid combustion reaction. Without nitrates, the fireworks wouldn’t ignite with such force or brilliance. They enable the rapid release of energy necessary to launch the firework and create the explosion that releases light and sound.
In short, nitrates are the silent enablers of celebration. Behind every boom and burst of color is a carefully calculated chemical reaction - and nitrates are what make it all possible.
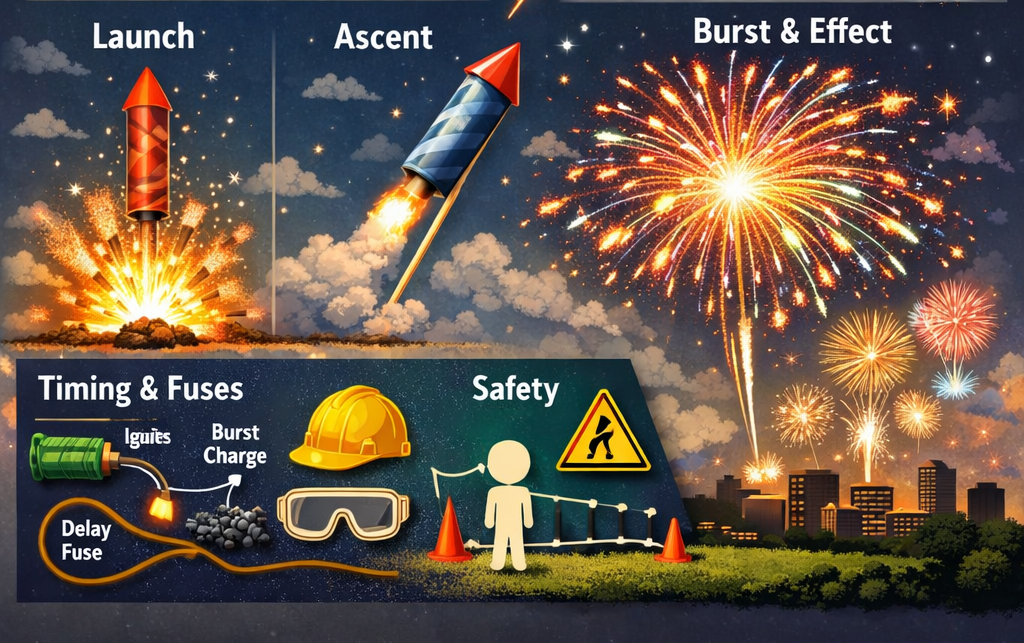
The Science of Fireworks: How They Work
From the outside, a firework may look like a simple tube or sphere, but inside lies a sophisticated chemical arrangement engineered for spectacle. A standard firework shell consists of several key parts: a lift charge to launch it into the air, a time-delay fuse to ignite the explosion at the right height, and a bursting charge packed with “stars” - small pellets containing metal salts and other compounds that produce color and effects.
At the core of firework chemistry is the oxidation-reduction (redox) reaction. This is a fast chemical process in which oxidizers release oxygen to support the burning of reducers (fuels), generating a sudden burst of heat and gas. This reaction drives the explosive force needed to project stars outward and trigger light and sound.
Each sensory effect like brilliant flashes, colorful sparks, and loud booms is the product of precise chemical tuning. The heat from combustion excites electrons in metal atoms; as they return to their normal state, they release energy in the form of light. The expanding gases create sound waves, and additives like aluminum or magnesium intensify both light and heat.
The Key Players: Oxidizers, Reducers, Binders
Every firework relies on a balanced mix of three essential components:
- Oxidizers: Provide oxygen. Examples include potassium nitrate, potassium chlorate and barium nitrate.
- Reducers (Fuels): Burn to release energy. Common choices are charcoal, sulfur and metals like aluminum.
- Binders: Hold everything together, usually dextrin or starch.
A precise ratio is vital. Too much oxidizer, and the firework may detonate unpredictably; too little, and it may fizzle out. The chemical harmony between these components ensures a safe, spectacular display.
Nitrates in Fireworks: The Chemical Backbone
Nitrates are the unsung heroes of pyrotechnics - essential to the chemistry that makes fireworks ignite, explode and dazzle. Chemically, nitrates are salts containing the nitrate ion (NO₃⁻) bonded with metal cations such as potassium (K⁺), sodium (Na⁺), or barium (Ba²⁺). Common examples include potassium nitrate (KNO₃), sodium nitrate (NaNO₃) and barium nitrate (Ba(NO₃)₂).
Nitrates function as
oxidizers, meaning they provide the oxygen needed for fuel to combust in the absence of atmospheric oxygen. This is crucial in the sealed environment of a firework shell, where a quick, self-contained chemical reaction must occur.
When heated, nitrate salts decompose to release oxygen and gaseous byproducts, fueling rapid exothermic reactions:
- 2 KNO₃ → 2 KNO₂ + O₂
- NaNO₃ → NaNO₂ + ½ O₂
- Ba(NO₃)₂ → BaO + 2 NO₂ + ½ O₂
This oxygen release feeds the combustion of fuels like carbon or sulfur, generating the explosive energy needed to launch fireworks and activate colorants. Because nitrate salts are stable, easy to store, and efficient oxygen donors, they remain the cornerstone of firework formulations.
Potassium Nitrate (Saltpeter): A Historic Oxidizer
Used in gunpowder since ancient China, potassium nitrate is one of the oldest known chemical oxidizers. Combined with sulfur and charcoal, it formed the basis of early fireworks and military explosives. Its high oxygen content and thermal stability made it ideal for sustained, controlled combustion - essential for propelling rockets and creating aerial effects.
Even today, KNO₃ is prized for its consistent performance and wide availability, serving as a reliable backbone in modern fireworks manufacturing.
Other Nitrates: Sodium and Barium Variants
While potassium nitrate drives combustion, other nitrate salts add brilliant colors to the show:
- Barium nitrate imparts a vivid green hue. Barium ions emit green light when excited by heat and the nitrate provides oxidizing power.
- Sodium nitrate, in contrast, creates a bright yellow glow. Its sodium ions release characteristic yellow light when heated, ideal for star effects.
These nitrate variants blend function with flair, combining oxidizing strength with vibrant colors to enhance the pyrotechnic experience.
Creating Colors: Nitrates and Metal Salts in Action
While nitrates supply the oxygen needed for combustion, they also play a vital supporting role in color formation. They act as the energetic foundation that allows metal salts like strontium, copper and barium to shine, quite literally.
When a firework explodes, the intense heat excites the electrons in the metal atoms. As these excited electrons return to their original energy levels, they release energy as visible light. The color depends on the type of metal ion and the energy gap between electron states. For example, strontium produces red, while copper yields blue.
Nitrate salts help facilitate this process by ensuring high, consistent combustion temperatures, which are essential for clear, vibrant colors. Without enough heat, the electrons won’t be sufficiently excited and colors may appear washed out or fail to show altogether.
This precise chemistry is why creating specific colors, like deep blue or purple, is more difficult: they require tightly controlled conditions and the right metal-nitrate combination.
Safety and Environmental Concerns of Nitrate Use
While fireworks bring excitement and wonder, the use of nitrates in pyrotechnics raises important safety and environmental concerns.
After a firework show, residual chemicals, including nitrates and metal salts, settle on the ground or enter waterways through rainfall. These residues can contribute to nitrate pollution, which promotes algal blooms in aquatic systems and disrupts ecosystems. Additionally, some nitrate byproducts, like nitrogen oxides (NOₓ), contribute to air pollution and respiratory issues, especially in densely populated areas.
From a safety perspective, nitrate compounds, especially in combination with fuels, are highly reactive and flammable. Improper storage, handling, or exposure to heat can lead to accidental ignition or even explosions. Some nitrates, such as barium nitrate, are also toxic to humans and animals, making safe disposal and protective gear essential in manufacturing and cleanup.
In response, scientists and manufacturers are developing “green” fireworks - formulations that reduce or replace nitrates with less hazardous oxidizers. These alternatives use nitrogen-rich compounds that burn cleaner, reduce smoke and limit toxic metal use, helping to minimize environmental impact without compromising performance.
Though nitrate-based fireworks remain the industry standard due to their effectiveness and affordability, the future of pyrotechnics may lie in
eco-friendly innovations that balance spectacle with sustainability.
Historical Evolution: From Ancient Nitrates to Modern Spectacles
The story of fireworks begins in ancient China, where alchemists around the 9th century discovered that a mixture of saltpeter (potassium nitrate), sulfur and charcoal could produce loud bangs and bright flashes when ignited. This early gunpowder formula was the foundation for both weaponry and celebration, giving rise to firecrackers and rudimentary rocket displays.
As fireworks spread to the Middle East and Europe through trade and conquest, the chemical compositions evolved. By the Renaissance, European pyrotechnicians were experimenting with metal salts and shaped shells, creating more sophisticated visual effects. Italian fireworks makers were especially influential, pioneering multicolored displays by incorporating new chemical elements.
Across cultures, the chemistry adapted to local materials and traditions. In India, firework displays featured bright flashes and smoke effects using local nitrates. In Japan, artisans developed intricate spherical shells “hanabi”, focused on aesthetic beauty, relying on potassium nitrate and precision-packed stars for symmetry and color.
Today, modern fireworks are the result of centuries of chemical refinement, combining ancient techniques with modern science. While nitrates still play a central role, today’s displays are brighter, safer and more diverse - proof of how one ancient oxidizer ignited a global tradition of celebration.
Modern Innovations in Firework Chemistry
Today’s fireworks are the product of centuries of experimentation and the pace of innovation is accelerating. Driven by concerns over safety, environmental impact, and visual sophistication, modern pyrotechnics are becoming safer, more colorful and more eco-friendly.
One major advance is the use of nanoparticles, which allow for more efficient combustion and sharper colors at lower temperatures. These particles, often made of aluminum or magnesium, enhance brightness and reduce the amount of oxidizer needed, helping minimize toxic byproducts. Additionally, modified nitrate compounds and cleaner-burning oxidizers are being tested to reduce smoke and fallout, especially in environmentally sensitive areas.
Manufacturers are also shifting toward greener formulations, replacing heavy metals with less toxic alternatives. For example, nitrogen-rich compounds can replace traditional nitrates in some fireworks, significantly reducing emissions of harmful gases like NOₓ.
Beyond chemistry, the art of fireworks has embraced technological precision. GPS, computer-controlled fuses, and wireless ignition systems now allow for exact timing and musical synchronization, turning fireworks into fully choreographed light and sound shows.
From ancient saltpeter to synchronized spectacles, modern firework chemistry reflects a blend of tradition, science and innovation, ensuring that the sky continues to dazzle for generations to come.
Chemistry That Captivates the Sky
Nitrates are the backbone of firework chemistry, providing the oxygen needed for combustion and enabling the vibrant displays that captivate audiences around the world. As we look to the future, the focus on safety, sustainability and innovative technologies promises even more dazzling, eco-friendly shows.
At
DECACHEM, we’re proud to supply high-quality nitrates that help power the pyrotechnics industry while ensuring safety and sustainability in every display. If you’re looking for reliable nitrate solutions,
contact us today to learn more about how we can support your chemical needs.
















